Enforcement of the Centers for Medicare & Medicaid Services’ COVID-19 vaccine mandate began January 27. CMS is taking a phased approach to enforcement to allow time to develop and implement policies, procedures and processes to ensure all staff are fully vaccinated for COVID-19 (or to document a delay or allowable exemption). With this phased approach, there are specific targets that must be met at 30 days (Phase 1), 60 days (Phase 2) and 90 days (full enforcement) with different enforcement dates for different groups of states.
Here's an explanation of the mandate and useful information for healthcare leaders about enforcement deadlines.
COVID-19 Vaccine Mandate in a Nutshell
The CMS COVID-19 vaccine mandate requires covered providers and suppliers to develop and implement policies and procedures by Phase 1 deadlines to ensure all staff are fully vaccinated for COVID-19. For hospitals, “staff” is broadly defined to include nearly all persons providing care, treatment or services. Enforcement is in three phases with tiered vaccination targets by the Phase 1 (80%), Phase 2 (90%) and full vaccination (100%) deadlines. By the full vaccination deadline, 100% of staff must have completed the vaccine series, have been granted a qualifying exemption or have been identified as having a temporary delay as recommended by the Centers for Disease Control and Prevention. Documentation verifying vaccination status, medical exemptions or delays may be sampled by surveyors. Additionally, healthcare leaders must have additional mitigation precautions and contingency plans in place for any staff who are not fully vaccinated and who continue to provide care, treatment or other services for the hospital and/or its patients.
How is “Staff” Defined by the COVID-19 Vaccine Mandate?
The CMS COVID-19 vaccine mandate applies to all staff who provide any care, treatment or other service for a hospital or its patients. “Staff” within the meaning of the rule includes employees, practitioners, students, trainees, volunteers, contracted staff and vendors. The COVID-19 vaccine mandate does not apply to staff who work exclusively offsite or remotely and who do not have any direct contact with patients or other “staff.” CMS notes that “[w]hen determining whether to require COVID-19 vaccination of an individual who does not fall into the categories established by this [rule], facilities should consider the frequency of presence, services provided, and proximity to patients and staff.”
What are the Minimum Requirements for COVID-19 Vaccine Policies and Procedures?
Hospitals must have policies and procedures developed and implemented by their Phase 1 deadline (see TABLE 1). These policies and procedures must include the following ten components:
- Process for ensuring staff have at least one vaccine dose prior to providing care, treatment or services.
- Process for ensuring that all staff are fully vaccinated by applicable deadlines.
- Process for ensuring the implementation of additional mitigation precautions for staff who are not fully vaccinated.
- Process for tracking vaccine status.
- Process for tracking booster dose(s).
- Process for requesting exemptions.
- Process for tracking exemptions.
- Process for verifying medical exemptions.
- Process for tracking delayed vaccinations.
- Contingency plans for staff who are not fully vaccinated.
How Will the COVID-19 Vaccine Mandate be Enforced?
A phased approach to enforcement began January 27 for hospitals in Group 1 states. Hospitals surveyed after their Phase 1 date should expect the surveyors to request two things: the organization’s COVID-19 vaccine policies and procedures, and a list of all staff with their vaccine status. From the staff list, surveyors will select a sample of direct care staff (both vaccinated and unvaccinated), contracted staff and direct care staff with documented exemptions. Excluding religious exemptions, surveyors will review and verify documentation of vaccination status and medical exemptions contained within records for the sampled staff.
According to the CMS Surveyor Guidance, if a surveyed facility does not meet the phased vaccination targets (see TABLE 1), is observably noncompliant with infection control practices, and has not developed or implemented one or more components of the policies and procedures, that hospital would earn a Condition Level deficiency with Immediate Jeopardy, which would result in penalties on an accelerated schedule.
TABLE 1. COVID-19 Vaccine Enforcement Dates and Staff Vaccination Targets
|
STATES |
PHASE 1 |
PHASE 2 |
FULL VACCINATION* |
|
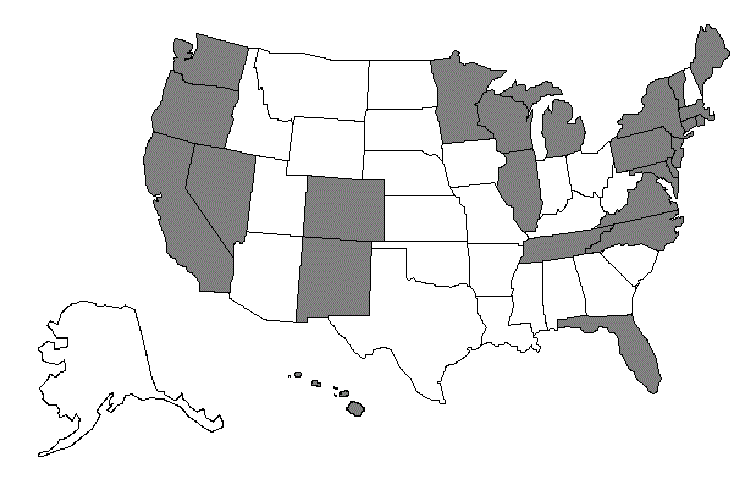
GROUP 1: CA, CO, CT, DE, DC, FL, HI, IL, ME, MD, MA, MI, MN, NV, NJ, NM, NY, NC, OR, PA, RI, TN, VT, VA, WA, WI
|
DEADLINE: Jan. 27, 2022 TARGETS:
|
DEADLINE: Feb. 28, 2022 TARGETS:
|
DEADLINE: March 28, 2022 TARGETS:
|
|
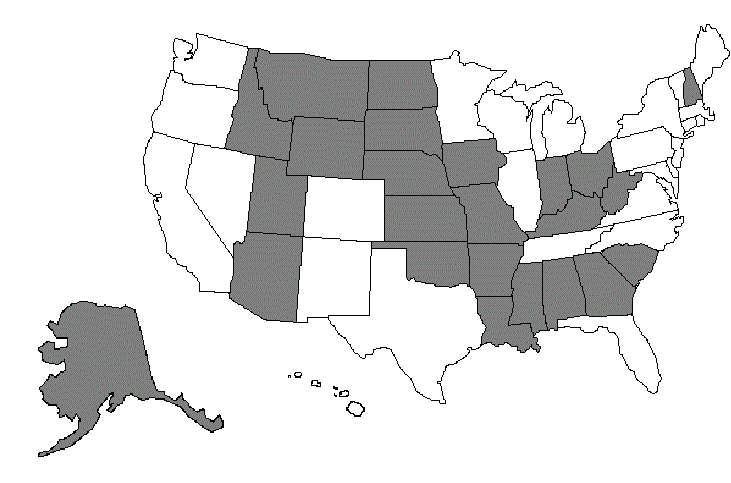
GROUP 2: AL, AK, AZ, AR, GA, ID, IN, IA, KS, KY, LA, MS, MO, MT, NE, NH, ND, OH, OK, SC, SD, UT, WV, WY
|
DEADLINE: Feb. 14, 2022 TARGETS:
|
DEADLINE: March 15, 2022 TARGETS:
|
DEADLINE: April 14, 2022 TARGETS:
|
|
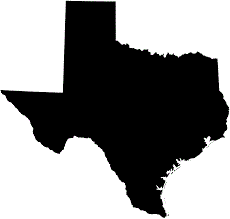
TEXAS: TX
|
DATE: Feb. 22, 2022 TARGETS:
|
DATE: March 21, 2022 TARGETS:
|
DATE: April 20, 2022 TARGETS:
|
*Staff are considered fully vaccinated if it has been two weeks or more since they completed a primary vaccination series for COVID-19; however, staff who have received the final dose of a primary vaccination series by the Phase 2 deadline are considered to have met the vaccination requirement for the purposes of calculating percentage of “staff” compliant with the vaccine mandate, But, these individuals would still be subject to the “additional precautions” requirement until 14 days had passed
NOTE: Staff who have been granted a qualifying exemption or whose vaccine series is temporarily delayed count for the purposes of compliance with 100% vaccination requirements under the rule.
Brent Ibata, PhD, JD, MPH, FACHE, is the system director for accreditation and quality assurance for Lee Health and teaches for the Master of Science in Regulatory Affairs program at Northeastern University. Ibata is the author of several COVID-19 pieces including a law journal article on “Crisis Standards of Care” and two ACHE COVID-19 blogs related to PPE and the OSHA ETS.